Why Clone Animals?
Given that we already knew from amphibian studies in the 1960s that nuclei were totipotent, why clone mammals? Many of the reasons are medical and commercial, and there are good reasons why these techniques were first developed by pharmaceutical companies rather than at universities. Cloning is of interest to some developmental biologists who study the relationships between nucleus and cytoplasm during fertilization, and to others who study aging (and the loss of totipotency that appears to accompany it). But cloned mammals are of special interest to the people and corporations concerned with creating protein pharmaceuticals.
Protein drugs such as human insulin, protease inhibitors, and clotting factors are difficult to manufacture. Because of immunological rejection problems, human proteins are usually much better tolerated by patients than proteins extracted from other species. Similarly, our bodies often reject proteins that have been synthesized by genetically engineered bacteria. The problem thus becomes how to obtain large amounts of human proteins. One of the most efficient ways to produce these proteins is to insert the human genes encoding them into the oocyte DNA of sheep, goats, or cows. Animals containing a gene from another individual (often of a different species)—a transgene—are called transgenic animals. A female sheep or cow made transgenic for the human protein gene might express this gene in her mammary tissue and secrete the protein in her milk (Figure 1; Prather 1991).
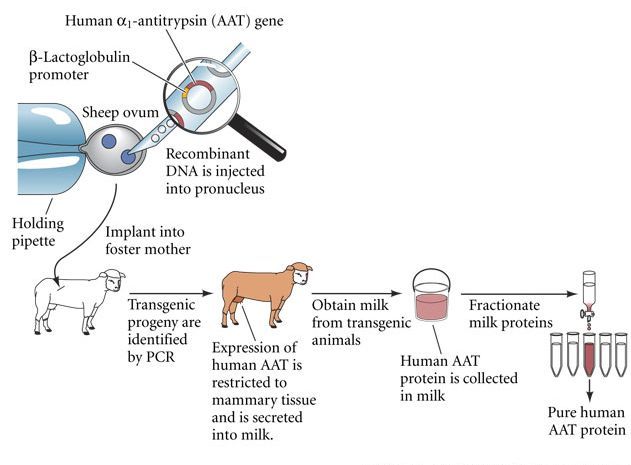
Figure 1 Cloning of transgenic mammals to produce protein pharmaceuticals. The structural gene for an important human protein (such as α1-antitrypsin; AAT) is linked to the regulatory region (promoter) of a sheep milk protein gene (such as that for casein or lactalbumin). This recombinant gene is injected into the pronucleus of a newly fertilized sheep egg, and the egg is implanted into a foster mother. Newborn sheep are screened for the presence of the human gene. When the transgenic sheep mature, the human gene should be expressed in the mammary gland and the protein secreted into the milk. From the milk one can then isolate large amounts of the protein for pharmaceutical use.
Producing transgenic sheep, cows, or goats is not an efficient undertaking, however. Only 20 percent of the treated eggs survive the technique. Of these, only about 5 percent actually express the human gene. And of those who do express the human gene, only half are female, and only a small percentage of these females actually secrete high levels of the human protein into their milk (plus the fact that it often takes years before they first produce milk). And then, when these rare animals die after several years of milk production, their offspring are usually not as good at secreting the human protein. But if pharmaceutical companies could clone such “elite transgenic animals,” the cloned females should all produce high yields of human protein in their milk. The economic incentives for such cloning are therefore enormous, with therapeutic proteins potentially becoming much cheaper for the patients who need them for survival. (Meade 1997). It was with this kind of scenario in mind that, shortly after the announcement of Dolly, the same laboratory announced the birth of Polly (Schnieke et al. 1997). Polly was cloned from transgenic fetal sheep fibroblasts that contained the gene for human clotting factor IX, a gene whose function is deficient in hereditary hemophilia.